As a child, you might dream one day of becoming an astronaut and, the next day, of becoming a ballet dancer — the possibilities are endless. Eventually, this wealth of choice is whittled down by external circumstances and internal interests. Similarly, precursor cells in early embryos make a series of stepwise ‘decisions’ governed by external forces and internal factors to generate the diverse array of cell types present in adult organisms1. Writing in Nature, Morita et al.2 show that the fate of cells that eventually make up mature hair follicles is determined by their positioning in concentric rings of nascent follicle structures during embryonic skin development. In this case, how and where cells begin their developmental journey ultimately decides their destination.
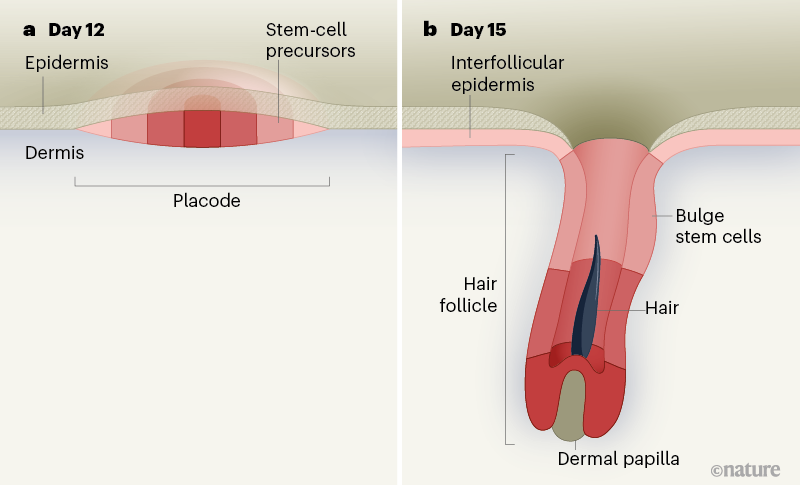
Hair-follicle development begins with a clean slate. The skin comprises two major components: the epidermis, a layer of epithelial cells that form a protective barrier against external insults; and the dermis, which contains cells called fibroblasts that support the skin3. Epithelial cells and fibroblasts seem to be equally able to contribute to and regulate the formation of hair follicles. Dermal fibroblasts induce the formation of placodes — cellular thickenings in the epithelial layer that eventually give rise to hair follicles4,5. Placodes develop as flat circles on the surface of the skin. How these flat disc structures eventually transform into a long, cylindrical 3D shape is a question of great interest not only to skin and hair researchers, but also to scientists investigating potential hair-replacement therapies that work through developmental mechanisms6,7.
This is where Morita and colleagues step in. The authors optimized a technique called live imaging to record the movement of cells in cultured skin tissue taken from mouse embryos, beginning at 11.5 days after conception. They imaged the skin samples for three to five days, in three dimensions and at a microscopic level, to produce time-lapse videos of hair-follicle development8. By playing these videos in reverse, the authors traced cells in fully grown hair follicles back to their embryonic placodal origins. They discovered that cells in early placode structures are organized into concentric zones, like the bullseye and surrounding rings of a target, such that the centre-most cells give rise to the bottom of the hair follicle, and cells at the outer edge of the placode remain at the surface of the skin, between follicles (Fig. 1). The downward growth from placode to fully developed hair follicle, which is enabled by the proliferation and movement of these cells over time, resembles the elongation of an extendable telescope; thus, the authors propose referring to this type of development as the “telescope model”.
Although this model is not familiar to hair biologists, the formation of cylindrical or other 3D-shaped appendages, such as legs and antennae, from flat surfaces was first described in the fruit fly Drosophila melanogaster9. Studies in flies have long informed biological principles in more-complex mammalian species; thus, although perhaps unsurprising, it is exciting that this telescope mechanism is evolutionarily conserved across species.
Adding further complexity to this system is the appearance of stem cells in the hair follicle as it develops. In adult animals, a reservoir of such cells resides in a part of the upper hair follicle called the bulge10,11 and is crucial for enabling the follicle to expand during the growth phase of the hair cycle, as part of normal tissue turnover throughout an animal’s life12. These stem cells can even repair injury to the rest of the epidermis12. The hair-follicle placode harbours predecessors to bulge stem cells, and the stem cells’ origin in the early placode was previously demonstrated by using protein markers expressed by the cells, such as the transcription factor SOX9, to track them. This approach suggested that, as the placode begins to extend downwards, bulge stem-cell precursors are born and remain superficial to the placode, before ultimately colonizing the bulge region13.
Morita and colleagues selectively labelled and isolated cells from early mouse hair follicles at the same time points as those analysed in their live-imaging experiments, to perform single-cell transcriptomics — gene-expression profiling of individual cells. Because single-cell transcriptomics allows a broad and unbiased survey of the genes and proteins expressed by each cell, it can be highly informative for ascribing cellular identity and for characterizing cellular dynamics during development2,8. Using this approach, Morita and colleagues identified a population of cells expressing bulge stem-cell markers at the earliest stages of placode development. Through mathematical modelling of cells at all assessed time points, the authors determined that these marker-expressing cells differentiate into bulge stem cells in the early placode6, long before the time point at which such differentiation was previously reported to occur12.
These stem-cell precursors expressed the gene that encodes SOX9, consistent with previous reports13. However, through further cell-lineage tracing and live imaging, these precursors were unequivocally demonstrated to originate from the previously unidentified peripheral ring zone of the early placode. This newly posited spatial localization will probably require a re-examination of previous findings related to bulge stem-cell precursors and reopen questions of the regulatory conditions necessary for the induction of this cell population.
Although the authors’ study makes huge strides in dissecting how different cell populations contribute to the mature hair follicle, it also invites many questions, the most compelling of which is how the concentric zones in the placode are established. It is likely that various elements contribute to the formation of these zones, such as diffusible factors secreted by other epidermal and dermal cells (including specialized dermal cells clustered directly below where placodes form14). By improving our understanding of hair-follicle development, future studies might unveil ways of generating hair follicles de novo that could eventually be used in hair-replacement therapies. However, unlike the routes taken by cells on their developmental journeys to their final destinations in the mature hair follicle, the pathway to such applications is still unclear.
Bagikan Berita Ini

















0 Response to "A 4D road map for the formation of hair follicles - Nature.com"
Post a Comment